A new plant for the production of sterile non-cytotoxic drugs and innovative products for diseases outside of the field of oncology with the objective to support complex and integrated portfolio of products, with new modalities of action and new effective therapies.
Our capabilities are designed to handle SMALL and LARGE MOLECULES, SOLVENT BASED FORMULATION and LIPID BASED FORMULATION for development, clinical and commercial supply.
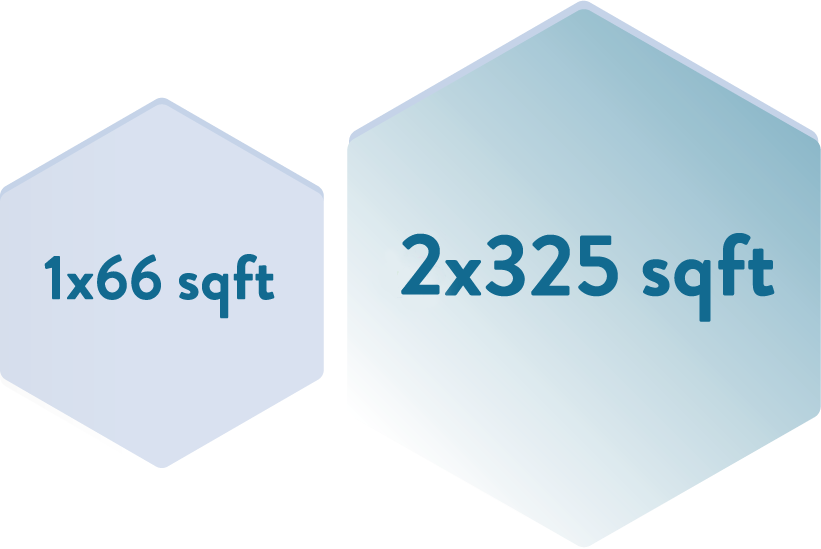
equipped to handle Liquid and Lyophilized vials from 2 ml to 100 ml with filling lines designed to manufacture products with short holding time and minimize shear stress.
DEDICATES AREAS FOR THAWING
STATIC AND DYNAMIC
TEMPERATURE CONTROL
DURING MANUFACTURING
CRYOGENIC LYOPHILIZERS
ENABLE A VERY ACCURATE AND EFFICIENT TEMPERATURE'S MANAGEMENT
DURING EACH STEP
AUTOMATIC LOADING & UNLOADING SYSTEM (ALUS)
DESIGNED FOR A RAPID INTRODUCTION OF THE VIALS INTO
THE LYO CHAMBER TO MINIMIZE PRODUCT'S EXPOSURE TO ROOM TEMPERATURE
100% CCIT BY FMS
VISUAL INSPECTION
MANUAL, SEMI-AUTOMATIC AND AUTOMATIC
SECONDARY PACKAGING available for Commercial supply and DEDICATED COLD STORAGE AREAS for DS, mAb, toxin and finish product (+2/+8°C; from -20°C up to -80°C) .