
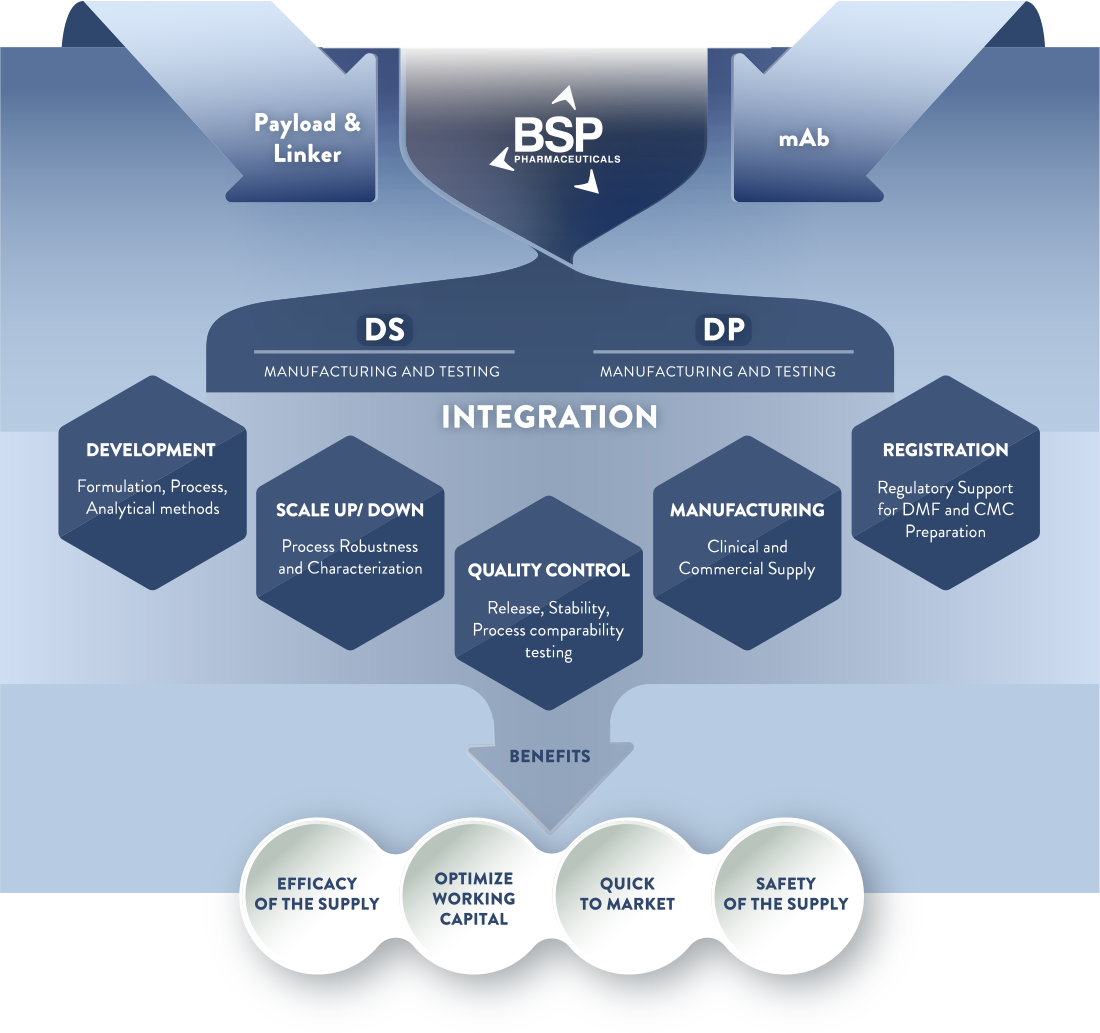
BSP developed capabilities to handle conjugation and fill finish, from the early development, to clinical up to the commercial scale, everything within the same Facility and under the same Quality system. We currently produce the majority of commercialized ADCs, supplying more than 80 countries globally (including EU, US, JP).
We support our clients to develop and commercialize new drug candidates, new conjugation technologies and platforms by offering our experience and technologies to support PAI, launch, and commercial manufacturing requirements.
CHECK FILL-FINISH CAPABILITIES FOR CYTOTOXIC
equipped with dedicate stainless steel or Single-Use disposable systems, customized for each products and process.
DEDICATED AREAS FOR STATIC AND DYNAMIC THAWING
TANGENZIAL FLOW FILTRATION (TFF) / DIAFILTRATION (DF)
CHROMATOGRAPHY
FILLING OF DISPOSABLE BAGS AND BOTTLING UNDER ISOLATOR
BSP’s commitment to support successful development of new ADCs has been strongly valued by innovators and product’s developer As first CDMO to offer an integrated supply chain for ADCs, manufacturing drug substance and drug product at the same site, since 2015 we received World ADC Award for Best ADC Contract Manufacturing Provider, in recognition of the efforts spent to promote advancement of the most promising candidates through clinical and commercial phase.


In 2021, BSP’s Founder, President and CEO, Aldo Braca, was also personally awarded in the category of “Individual input to the field”, for the major role in sustaining the recent growth in the number of the approved ADC products.
