

The passion for what we do, the sense of belonging to the company community, the desire to actively take part in innovation, make our work stimulating and rich in challenges. A breakthrough in the scientific community is also a breakthrough for us, a result that we have contributed to achieving as the target of a process managed by BSP. To offer our experience and professional skills to the pharmaceutical field is not only our job, but above all an ethical commitment.
Our company organization enables us to face all production and scientific challenges with the utmost flexibility, assigning a dedicated team to each project, becoming as an extension of the Client.
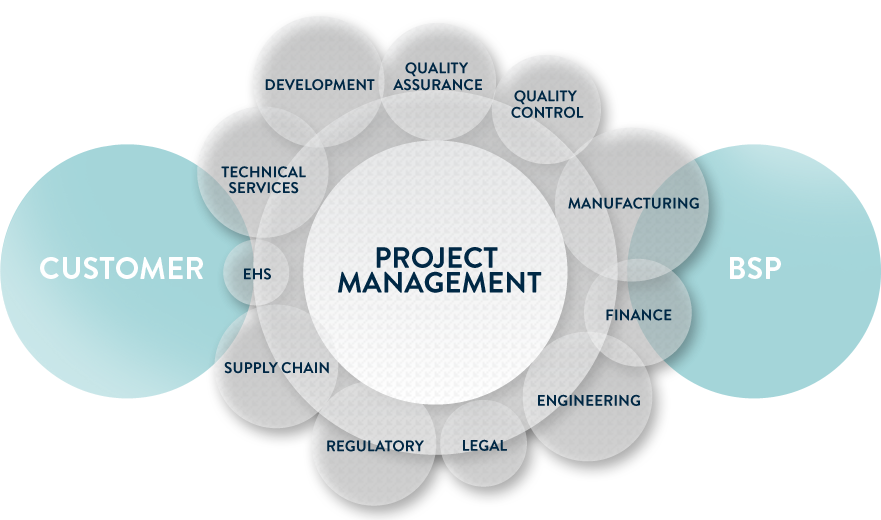
The Project Management is the hub of the process, integrating BSP excellences and acting as a link between the Client and our specialized departments.
A PROMISE OF RELIABILITY TO THE CLIENT, RESPECT FOR PATIENTS AND ENVIRONMENT, ETHICAL COMMITMENTS ARE THE FOUNDATIONS ON WHICH BSP HAS BASED ITS INNOVATION STRATEGY.
With its services ranging from the development of new formulations for oral and injectable products to clinical and commercial supply, BSP has forged an important role in the production of drugs for the multinational pharmaceutical industries and small biotech companies alike.
The new technologies and high containment plants installed at BSP, create a bridge between the most innovative research projects and the patient.
Our technological development consists in new anticancer drugs, ranging from new generation small molecules to ADC products, liposomal formulations, micro and nano-emulsions.
THE BSP PLANT IS DESIGNED TO ENSURE HIGH LEVEL CONTAINMENT THROUGHOUT THE DEVELOPMENT, PRODUCTION AND QUALITY CONTROL OF ANTICANCER PRODUCTS, INCLUDING HIGH POTENT CYTOTOXIC COMPOUNDS.
The containment policy allows the preparation of anticancer products with an OEL lower
than 10 nanograms/m3 without any PPE (personnel protection equipment).
Isolators, cRABS, containment valves
to surrounding areas
of differential pressures and related alarm system
of exhaust air
segregation, inactivation and disposal
SETTING WORLD CLASS STANDARDS OF QUALITY TO SAFEGUARD PATIENTS.
Quality deeply permeates all operations at BSP. Each department acts with the awareness of fulfilling this approach responsibly in an integrated and robust Quality System.
The BSP Quality System is designed to support different processes by assuring compliance with the most restrictive regulatory rules both in the clinical and commercial supply.